 Dr Reddys Laboratories, an integrated global pharmaceutical company, announced today that it has launched Levalbuterol Inhalation Solution in the US market. The solution has USP 0.31 mg/3 mL, 0.63 mg/3 mL, 1.25 mg/3 mL unit-dose Vials, a therapeutic equivalent generic version of XOPENEX inhalation solution is approved by the United States Foods & Drug Administration (USFDA).
Dr Reddys Laboratories, an integrated global pharmaceutical company, announced today that it has launched Levalbuterol Inhalation Solution in the US market. The solution has USP 0.31 mg/3 mL, 0.63 mg/3 mL, 1.25 mg/3 mL unit-dose Vials, a therapeutic equivalent generic version of XOPENEX inhalation solution is approved by the United States Foods & Drug Administration (USFDA).
 The XOPENEX inhalation solution brand and generic combined had US sales of approximately USD 269.7 million MAT for the most recent twelve months ending in June 2014 according to MIS Health.
The XOPENEX inhalation solution brand and generic combined had US sales of approximately USD 269.7 million MAT for the most recent twelve months ending in June 2014 according to MIS Health.
Dr Reddy's Levalbuterol Inhalation Solution, USP 0.31 mg/3 mL, 0.63 mg/3 mL, 1.25 mg/3 mL are available in Unit-Dose Vials of (5x3 mL) carton of 25. Levalbuterol Inhalation Solution, USP is only for use with a nebulizer.
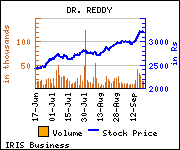
Shares of the company declined Rs 2.05, or 0.06%, to trade at Rs 3,206.25. The total volume of shares traded was 23,253 at the BSE (11.36 a.m., Wednesday).