 Dr Reddy's Laboratories, an integrated global pharmaceutical company, has launched Duloxetine Delayed-Release Capsules USP 20 mg, 30mg and 60 mg, a therapeutic equivalent generic version of CYMBALTA in the US market on Jun. 26, 2014.
Dr Reddy's Laboratories, an integrated global pharmaceutical company, has launched Duloxetine Delayed-Release Capsules USP 20 mg, 30mg and 60 mg, a therapeutic equivalent generic version of CYMBALTA in the US market on Jun. 26, 2014.
 Dr Reddy's ANDA is approved by the United States Food & Drug Administration (USFDA).
Dr Reddy's ANDA is approved by the United States Food & Drug Administration (USFDA).
The CYMBALTA brand and generic had U.S. sales of approximately USD 5.04 billion MAT for the most recent twelve months ending in April 2014.
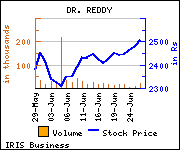
Shares of the company gained Rs 33.15, or 1.32%, to trade at Rs 2,537.95 at the BSE (10.19 a.m., Friday).