 Pharma major Lupin announced today that it has received final approval for its Rifabutin Capsules USP, 150 mg from the United States Food and Drugs Administration (FDA) to market a generic version of Pharmacia and Upjohn Company's Mycobutin capsules 150 mg. Lupin Pharmaceuticals (LPI), the company's US subsidiary would commence marketing the product shortly.
Pharma major Lupin announced today that it has received final approval for its Rifabutin Capsules USP, 150 mg from the United States Food and Drugs Administration (FDA) to market a generic version of Pharmacia and Upjohn Company's Mycobutin capsules 150 mg. Lupin Pharmaceuticals (LPI), the company's US subsidiary would commence marketing the product shortly.
 Lupin's Rifabutin capsules, 150 mg are generic equivalent of Pharmacia And Upjohn company's Mycobutin capsules 150 mg.
Lupin's Rifabutin capsules, 150 mg are generic equivalent of Pharmacia And Upjohn company's Mycobutin capsules 150 mg.
Lupin's Rifabutin capsules USP, 150 mg is indicated for the prevention of disseminated Mycobacterium avium complex (MAC) disease in patients with advanced HIV infection.
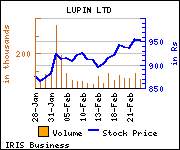
Shares of the company gained Rs 4.05, or 0.42%, to trade at Rs 958. The total volume of shares traded was 51,416 at the BSE (2.57 p.m., Wednesday).