 Ranbaxy Laboratories, one of the leading pharma players, today announced that the company has received approval from the US Food and Drug Administration (USFDA) to manufacture and market Fenofibrate Capsules USP, 43 mg and 130 mg. The office of generic drugs, US Food and Administration has determined the Ranbaxy formulations to be bioequivalent and have the same therapeutic effect as that of the reference listed drug Antara Capsules, 43 and 130 mg respectively of Lupin Atlantis.
Ranbaxy Laboratories, one of the leading pharma players, today announced that the company has received approval from the US Food and Drug Administration (USFDA) to manufacture and market Fenofibrate Capsules USP, 43 mg and 130 mg. The office of generic drugs, US Food and Administration has determined the Ranbaxy formulations to be bioequivalent and have the same therapeutic effect as that of the reference listed drug Antara Capsules, 43 and 130 mg respectively of Lupin Atlantis.
 Total annual market sales for Fenofibrate Capsules USP, 43 mg and 130 mg were USD 56 million (IMS-MAT: September 2014). Fenofibrate Capsules are indicated for Primary Hypercholesterolemia and Mixed Dyslipidemia. In addition, it is indicated for severe Hypertriglyceridemia.
Total annual market sales for Fenofibrate Capsules USP, 43 mg and 130 mg were USD 56 million (IMS-MAT: September 2014). Fenofibrate Capsules are indicated for Primary Hypercholesterolemia and Mixed Dyslipidemia. In addition, it is indicated for severe Hypertriglyceridemia.
''We are pleased to receive approval for these two strengths of Fenofibrate Capsules, which represents a welcome addition to our generic portfolio of products and offer an alternative in strengths and dosage forms for this molecule to patients and healthcare professionals. The product will be manufactured at Ohm Laboratories in our US facility located in New Brunswick, New Jersey and launched immediately thereafter,'' according to Dan Schober, vice president, trade sales, Ranbaxy.
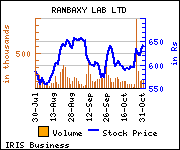
Shares of the company gained Rs 15.9, or 2.48%, to trade at Rs 657.05. The total volume of shares traded was 162,818 at the BSE (2.48 p.m., Wednesday).