 Lupin, one of the leading pharmaceutical companies in India, announced today that it has received final approval for its Celecoxib capsules, 50 mg from the United States Food and Drugs Administration (FDA) to market a generic version of G. D. Searle LLC's (a subsidiary of Prizer Inc.) Celebrex capsules 50 mg.
Lupin, one of the leading pharmaceutical companies in India, announced today that it has received final approval for its Celecoxib capsules, 50 mg from the United States Food and Drugs Administration (FDA) to market a generic version of G. D. Searle LLC's (a subsidiary of Prizer Inc.) Celebrex capsules 50 mg.
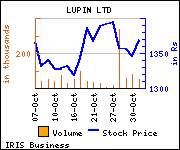 Lupin also received tentative approvals for its capsules Celecoxib capsules 100 mg, 200 mg and 400 mg strengths from the FDA.
Lupin also received tentative approvals for its capsules Celecoxib capsules 100 mg, 200 mg and 400 mg strengths from the FDA.
Lupin came into existence following the amalgamation of Lupin laboratories with Lupin Chemicals. Its activities include pharmaceuticals, bulk drugs and formulations, fermentation, bio-technology, natural products and agro chemicals.
Shares of the company declined Rs 5.45, or 0.4%, to trade at Rs 1,363.65. The total volume of shares traded was 21,737 at the BSE (11.27 a.m., Monday).