 Jubilant Life Sciences, an integrated Pharmaceuticals and Life Sciences Company, has received Abbreviated New Drug Application (ANDA) final approval from the US Food and Drug Administration (US FDA) for Montelukast Sodium Chewable Tablets, 4 mg and 5 mg, the generic version of Singulair Chewable Tablets (of Merck), which is used for the treatment of asthma and to relieve symptoms of seasonal allergies. The total market size for Montelukast Tablets as per IMS is USD 83 million per annum.
Jubilant Life Sciences, an integrated Pharmaceuticals and Life Sciences Company, has received Abbreviated New Drug Application (ANDA) final approval from the US Food and Drug Administration (US FDA) for Montelukast Sodium Chewable Tablets, 4 mg and 5 mg, the generic version of Singulair Chewable Tablets (of Merck), which is used for the treatment of asthma and to relieve symptoms of seasonal allergies. The total market size for Montelukast Tablets as per IMS is USD 83 million per annum.
 As on Dec. 31, 2014, Jubilant Life Sciences had a total of 781 filings for formulations of which 333 have been approved in various regions globally. This includes 72 ANDAs filed in the US, of which 35 have been approved and 46 Dossier filings in Europe. So far, we have received 10 ANDA approvals during FY 2015.
As on Dec. 31, 2014, Jubilant Life Sciences had a total of 781 filings for formulations of which 333 have been approved in various regions globally. This includes 72 ANDAs filed in the US, of which 35 have been approved and 46 Dossier filings in Europe. So far, we have received 10 ANDA approvals during FY 2015.
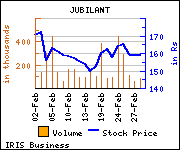
Shares of the company declined Rs 0.35, or 0.22%, to settle at Rs 159.30. The total volume of shares traded was 43,116 at the BSE (Monday).