 Aurobindo Pharma announced that the company has received final approvals from the US Food & Drug Administration (USFDA) to manufacture and market Azithromycin for Injection USP, 500mg /vial (ANDA 203294).
Aurobindo Pharma announced that the company has received final approvals from the US Food & Drug Administration (USFDA) to manufacture and market Azithromycin for Injection USP, 500mg /vial (ANDA 203294).
 Azithromycin for Injection USP, 500mg /vial is bioequivalent and therapeutically equivalent to the reference listed drug product (RLD) Zithromax® (Azithromycin for Injection) 500mg/vial of Pfizer, Inc.
Azithromycin for Injection USP, 500mg /vial is bioequivalent and therapeutically equivalent to the reference listed drug product (RLD) Zithromax® (Azithromycin for Injection) 500mg/vial of Pfizer, Inc.
Azithromycin for injection, USP is a macrolide antibacterial drug indicated for the treatment of patients with infections caused by susceptible strains of the designated microorganisms in the conditions such as Community-Acquired Pneumonia and Pelvic Inflammatory Disease Aurobindo now has 12 ANDAs (represented by 9 product classes) approved out of Unit IV formulation facility in Hyderabad, India for manufacturing general injectable products and will be marketed and sold by Aurobindo's wholly owned subsidiary AuroMedics Pharma LLC.
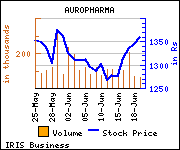
Shares of the company gained Rs 16.1, or 1.18%, to trade at Rs 1,375.00. The total volume of shares traded was 31,177 at the BSE (10.29 a.m., Monday).